CTSI SRC Review Requirements
Prior to Institutional Review Board (IRB) review, Indiana CTSI Scientific Review Committee (SRC) approval is required for new studies that meet the following criteria or when deemed necessary as an IRB provision for approval.
- Requires convened biomedical IRB review (full Board) or is considered flexible expedited categories 400(b) and/or 700(b),
- 400(b): Exercise interventions involving healthy individuals not involving invasive procedures, except collection of blood as described in Categories 2 and 200.
- 700(b): Food and diet interventions, excluding research involving medical foods as such term is defined by the FDA.
- No previous peer review process
- Peer review may include review of the protocol by an external funder, such as a federal agency or established not-for-profit research foundation, or protocol development by a commercial sponsor, such as a pharmaceutical or medical device company. Indiana CTSI SRC review is not required for studies that undergo Cancer Center SRC review.
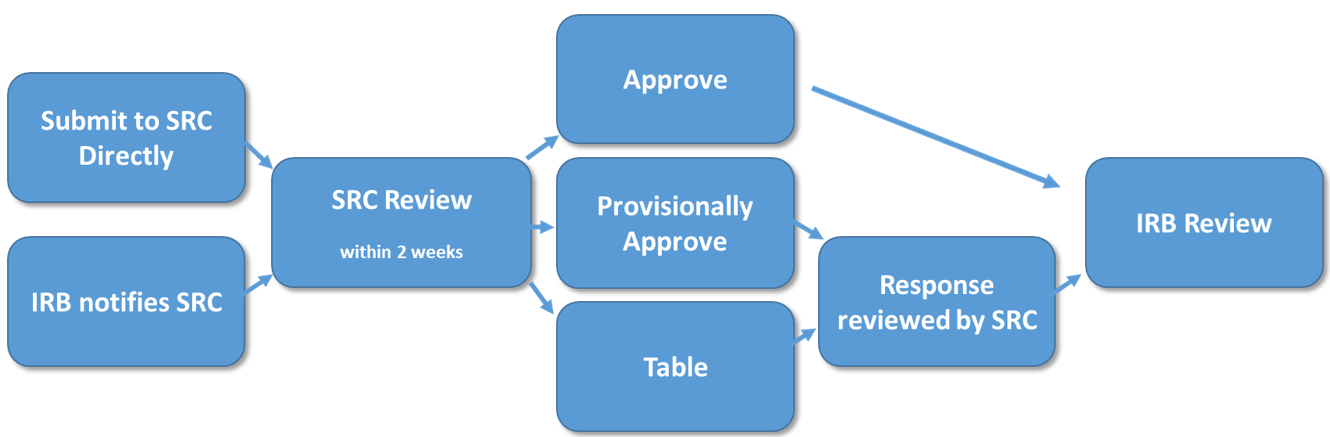
Investigators should send protocols to the Scientific Review Committee directly at ctsisrc@iupui.edu prior to IRB submission. The SRC will review new studies within two weeks. Human Subjects Office and IRB review will not occur until after SRC review and approval.
CTSI SRC Review Criteria
The instructions found in the IU IRB Protocol Template may facilitate protocol development aligned with the SRC review criteria. The Scientific Review Committee will review studies under the following review criteria:
- Objectives: Clearly stated specific aims aligned with well-defined endpoints and appropriate study design.
- Scientific Merit/Background and Rationale: Justification for conducting the study; results of similar or pilot data; current literature cited.
- Design: Clearly describes how stated objectives will be achieved, methods to acquire data, and strategies to overcome anticipated barriers. Addresses randomization, minimization of bias, patient follow-up, and blinding (if applicable).
- Eligibility Criteria: Specific inclusion/exclusion requirements; stratification factors if applicable.
- Outcome Characteristics and Endpoint Definitions: Clearly defined primary and secondary endpoints/outcomes aligned with the stated objectives.
- Statistical Analysis and Sample Size: Appropriate and adequate study design statistical analysis plan. Prospective analysis plan, including sample size justification to achieve study objectives and plans to minimize missing data.
- Data Management: Practices and procedures to manage data analysis, quality, cleaning, and storage.
- Principal Investigator and Study Site Qualifications and Resources: Has the necessary skills, experience, time, and resources to ensure that the study can be successfully completed, including identification of personnel to provide statistical computations and statistical expertise. A plan to register protocol with clinicaltrials.gov.
If the Scientific Review Committee finds that each criterion is Present/Acceptable, the committee will approve. If any criteria are Absent or deemed Not Acceptable, the Scientific Review Committee will return comments to the Principal Investigator (PI) for revisions. The PI must address all Scientific Review Committee comments by making recommended revisions or providing further justification for resubmission to the committee. When resubmitting to the Scientific Review Committee, respond point-by-point to comments and revise the protocol using tracked changes. Once the committee approves the revised protocol, it will notify the PI, at which time the study can be submitted to the IRB for review.
